
Other uses: Manganese has various other industrial applications, including in the production of alloys, chemicals, and as a nutritional supplement in animal feed and human diets.

Fertilizers: Manganese is used as a component in fertilizers to improve plant growth.Pigments: Manganese compounds are used as pigments in paints, ceramics, and glass.Batteries: Manganese is used in the production of batteries, including alkaline and rechargeable batteries, due to its high electrochemical activity.Steel production: Manganese is an essential element in the production of steel, where it acts as a deoxidizer and desulfurizer, improving the strength and toughness of the steel.Distribution: Manganese is widely distributed around the world, with major deposits found in countries such as South Africa, Australia, Brazil, China, and Gabon.Abundance: Manganese is the 12th most abundant element in the Earth’s crust, occurring in numerous minerals, rocks, and soils.Complex formation: Manganese has a strong ability to form complexes with other compounds, which makes it useful in various chemical processes.Magnetism: Some manganese compounds exhibit magnetic properties, and manganese is used in the production of ferromagnetic alloys.Reactivity: Manganese is a relatively reactive metal, readily forming compounds with oxygen, sulfur, and other elements.These oxidation states give manganese its versatile chemical reactivity. Oxidation states: Manganese can exist in various oxidation states, with the most common ones being +2, +3, +4, +6, and +7.Crystal structure: Manganese has a body-centered cubic crystal structure.Density: The density of manganese is about 7.43 grams per cubic centimeter.Melting and boiling point: The melting point of manganese is 1,246 degrees Celsius (2,275 degrees Fahrenheit), and its boiling point is 2,061 degrees Celsius (3,742 degrees Fahrenheit).Appearance: Manganese is a hard, brittle, silvery-gray metal.Some basic properties of manganese include: Manganese is known for its diverse oxidation states, which range from +2 to +7, and its ability to form numerous compounds with different properties. It is a transition metal, belonging to Group 7 in the periodic table. Manganese is a chemical element with the symbol Mn and atomic number 25. Pure (99.9%) manganese fragments, refined by electrolysis, next to a 1 cm³ cube Definition and basic properties of manganese Therefore, proper safety measures and regulations are necessary for handling and using manganese in industrial processes. Inhalation of manganese dust or fumes can lead to respiratory issues, and chronic exposure to manganese has been associated with neurological disorders known as manganism. Additionally, manganese is used as a pigment in paints, as a component in fertilizers to improve plant growth, and as a nutritional supplement in animal feed and human diets.ĭespite its numerous industrial applications, manganese can also have detrimental effects on human health and the environment when present in high concentrations. Manganese is also used in the production of batteries, such as alkaline and rechargeable batteries, due to its high electrochemical activity. One of its primary uses is in the production of steel, where it acts as a deoxidizer and desulfurizer, improving the strength and toughness of the steel. Manganese has many important applications in modern society. These oxidation states give manganese its versatile chemical properties, making it useful in various industrial processes. Manganese has several different oxidation states, with the most common ones being +2, +3, +4, +6, and +7. It is also present in trace amounts in plants, animals, and human tissues. In nature, manganese is typically found in the form of manganese oxides, which are abundant in soil, rocks, and minerals. The name “manganese” is derived from the Latin word “magnes,” which means magnet, as some manganese compounds exhibit magnetic properties. Manganese was first isolated as a distinct element in 1774 by Swedish chemist Johan Gottlieb Gahn, although its presence in ores and minerals had been known for centuries. It is also used in various industrial applications, such as the production of steel, batteries, and fertilizers.
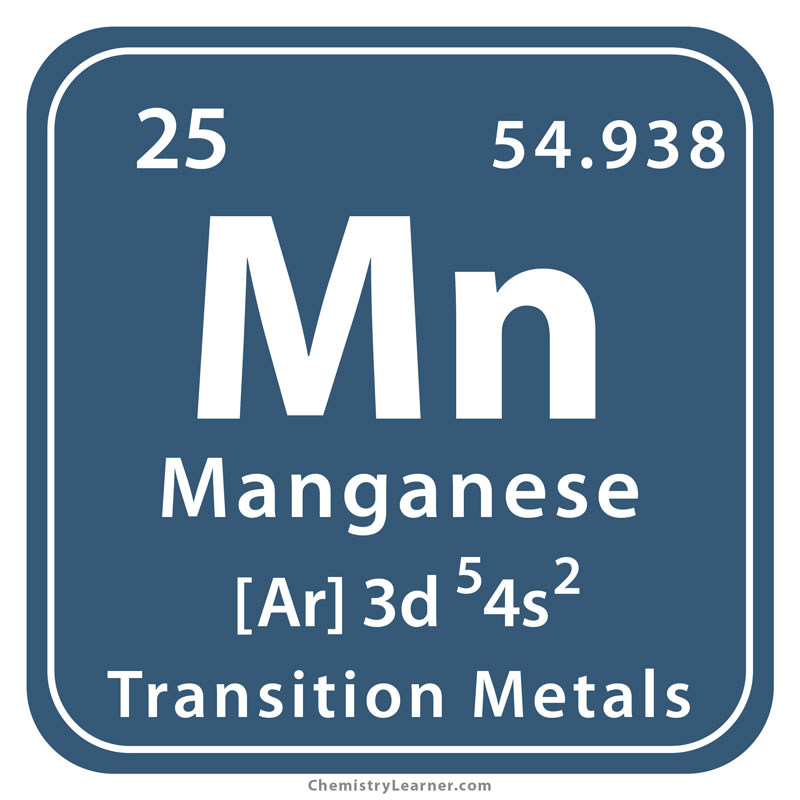
Manganese is an essential trace element that plays a crucial role in many biological processes, including metabolism, bone formation, and antioxidant function. It is a hard, brittle, silvery-gray metal that is commonly found in the Earth’s crust.


 0 kommentar(er)
0 kommentar(er)
